Diagnostics Devices
At Meril Diagnostics, we embrace technologies and innovation combined with a customer-centric approach to deliver clinically researched diagnostic products with precession and accuracy covering the full spectrum of central labs, hospitals and home testing.
Meeting the demands of changing landscape in diagnostics development including the over-the-counter diagnostics products requires a next generation service provider. Meril’s scientific expertise and economical solutions offer greater peace of mind among healthcare professionals, such as State-of-art Diagnostics, Domain Expertise, Commitment to Quality, Delivering value, Focus on service, Robust (Global) footprint and Global recognition.



Support an Education Ecosystem
Comitment to Quality
Ever-evolving MedTech Solutions
Manufacturing prowess
Meril believes that the delivery of the latest, most-relevant medical equipment requires ultra-modern manufacturing facilities. For this purpose, we have the cardiovascular, orthopaedic, diagnostic, endo-surgery business verticals along with a robust R&D foundry. We currently manufacture more than 100 products across the four verticals mentioned earlier.
Meril Park sprawls 250,000 square feet. It is our dedicated manufacturing and R&D facility for in-vitro diagnostic, orthopaedic, endo-surgery and cardio vascular solutions. Meril’s cardio vascular manufacturing comprises 40,000 square feet of built-up area along with 10,000 square feet of clean rooms.
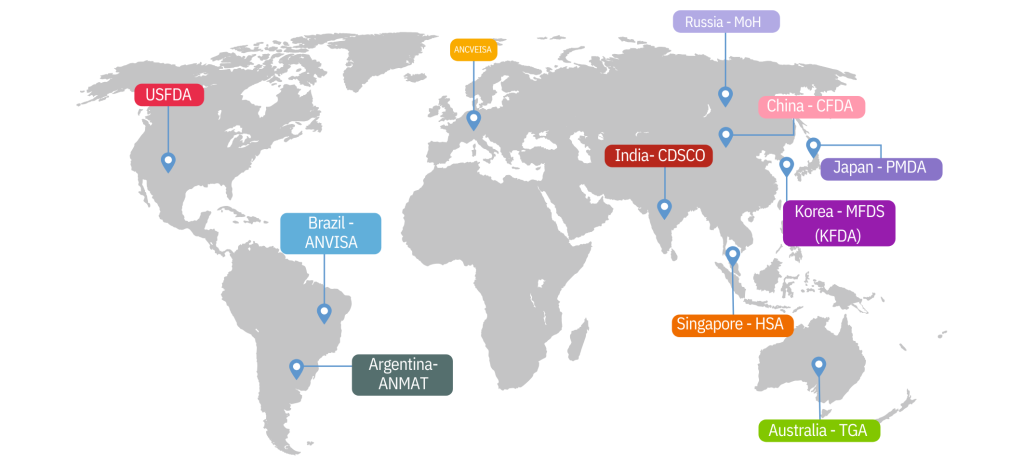
Our manufacturing facilities strictly adhere to cGMP guidelines, are ISO 13485:2016 certified and are DCG (I)-approved. Meril is equipped to handle complete in-house manufacturing for coronary drug eluting stents and balloon catheters. Our facilities handle laser cutting, descaling/annealing, electro polishing, drug coating–DES PTCA balloon manufacturing, microbiology, quality control and assurance. CE marked and in several instances approved by USFDA, we have also been awarded the following certifications: Canadian-CMDCAS, Japan-PMDA, China – CFDA, Korea – KFDA, Australia – TGA, Brazil – ANVISA, ANMAT, Russia MoH, Singapore HSA and other global regulatory bodies.
Purpose:
To affirm our relentless affinity with scientific progress, and uncover solutions to some of medicine’s most formidable challenges.
Vision:
To address the most pressing healthcare needs of this age and add more to the lives of countless people, across the world.
Mission:
To continually seek excellence in bringing forth innovative MedTech Solutions, in the service of alleviating human suffering.